Leaky Gut Syndrome
What Science Actually Says About Intestinal Permeability

- Published on: 29/Dec/2025
- Posted By: Arka Health
Introduction: From controversy to clinical clarity
For many years, the phrase leaky gut syndrome sat uncomfortably between patient experience and mainstream medicine. Individuals suffering from chronic digestive discomfort, fatigue, joint pain, brain fog, skin issues, or autoimmune conditions were often told their tests were normal and that no clear explanation existed. The term itself was frequently dismissed as vague or unscientific.
Over the last two decades, that perception has changed dramatically.
Modern research has confirmed that intestinal permeability, the scientific term for leaky gut, is a measurable and biologically regulated process. The intestinal barrier is no longer viewed as a static wall, but as a dynamic, selectively permeable interface that determines immune tolerance, inflammation, and systemic health.
Today, increased intestinal permeability is recognised as a contributing factor in autoimmune disease, metabolic disorders, neuroinflammation, and chronic digestive conditions. Understanding leaky gut is no longer about alternative theories. It is about molecular biology, immune signaling, and epithelial integrity.
This article explains what science actually says about leaky gut syndrome, how it develops, why it affects the entire body, and how a structured, evidence-based approach can restore gut barrier function.
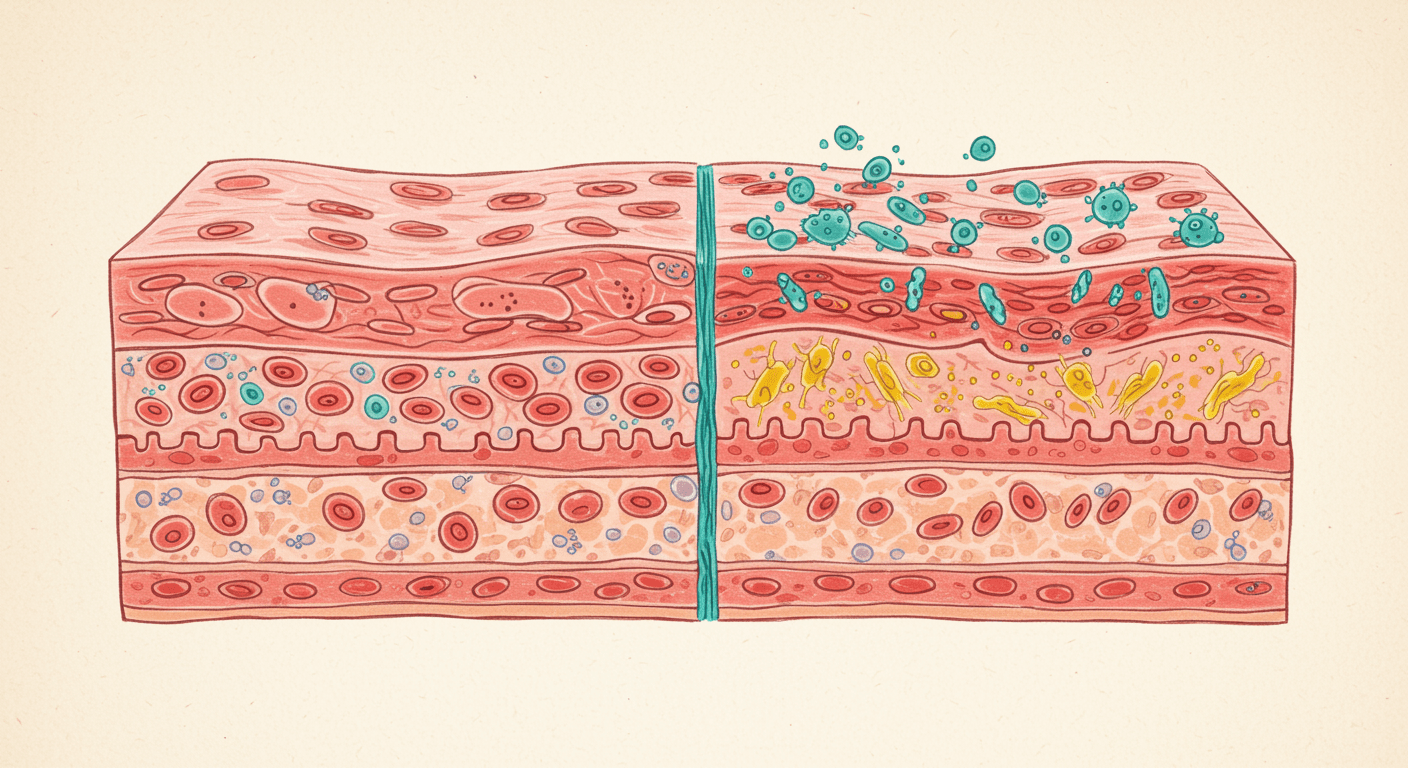
The intestinal barrier: a highly engineered defense system
The gastrointestinal tract covers an enormous surface area and faces constant exposure to food antigens, microbes, and toxins. To manage this, the body relies on a multi-layered intestinal barrier designed to allow nutrient absorption while preventing harmful translocation into the bloodstream.
This barrier consists of four integrated components.
The microbiological layer
The gut microbiome forms the first line of defense. Beneficial bacteria occupy ecological niches along the intestinal lining and prevent pathogenic organisms from attaching. These microbes also ferment dietary fiber to produce short-chain fatty acids such as butyrate.
Butyrate plays a critical role in maintaining epithelial health. It fuels intestinal cells and strengthens tight junction integrity. When microbial diversity declines, this protective signaling weakens, leaving the barrier vulnerable.
The mucus and chemical layer
Beneath the microbiota lies a structured mucus layer composed of mucin proteins. This layer prevents bacteria from contacting epithelial cells directly and acts as a reservoir for antimicrobial peptides and secretory IgA.
Secretory IgA binds bacteria and food antigens, neutralising them before they reach the intestinal wall. Reduced mucus thickness or low IgA levels often represent the earliest stage of barrier dysfunction.
The epithelial and tight junction layer
The intestinal epithelium is a single-cell-thick lining that separates the gut lumen from systemic circulation. These cells are connected by tight junction proteins, including claudins, occludin, and zonula occludens.
Tight junctions act as gatekeepers. They regulate what passes between cells and open briefly under controlled conditions to allow water and small nutrients through. In leaky gut, these gates become dysregulated and remain excessively open.
The immune layer
Directly beneath the epithelium sits the gut-associated lymphoid tissue, which contains the majority of the body’s immune cells. When the barrier functions properly, immune tolerance is maintained. When permeability increases, immune activation becomes chronic.
The molecular science behind leaky gut
Leaky gut does not occur through passive damage alone. It is an actively regulated process, driven by specific molecular pathways.
Zonulin and tight junction control
Zonulin is a human protein that modulates tight junction opening. Its physiological role is protective, allowing temporary permeability to flush pathogens from the gut. Problems arise when zonulin signaling becomes chronically activated.
Triggers such as gliadin, bacterial components, and inflammation stimulate zonulin release. Zonulin then activates intracellular signaling cascades that cause tight junction disassembly, increasing paracellular permeability.
In healthy individuals, this process is short-lived. In susceptible individuals, tight junctions fail to reassemble properly.
Inflammatory cytokines and barrier breakdown
Chronic inflammation worsens permeability through cytokines such as TNF-alpha and interferon-gamma. These cytokines increase cytoskeletal contraction within epithelial cells and reduce tight junction protein expression.
This creates a self-reinforcing loop where permeability drives inflammation, and inflammation further degrades the barrier.
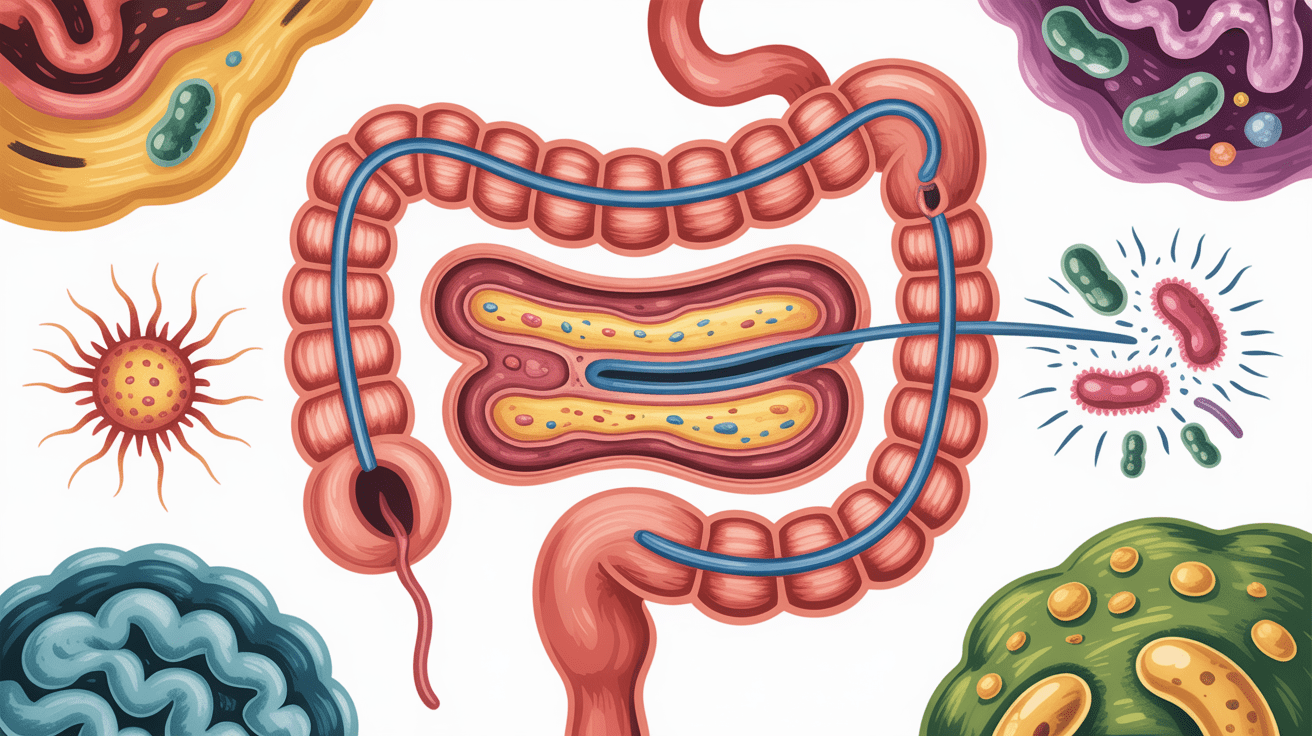
What causes leaky gut syndrome
Leaky gut is rarely caused by a single factor. It usually develops from cumulative environmental and physiological stressors.
Diet-related triggers
Certain dietary components are known to disrupt barrier integrity.
Gluten, specifically gliadin, stimulates zonulin release in all humans. In individuals with impaired repair mechanisms, this response becomes prolonged.
Ultra-processed foods containing emulsifiers damage the mucus layer and promote bacterial encroachment.
Excess alcohol directly injures epithelial cells and destabilises tight junction proteins.
Low-fiber diets deprive beneficial bacteria of fuel, reducing butyrate production needed for epithelial repair.
Chronic psychological stress
Stress has a direct physiological impact on gut permeability. Stress hormones activate mast cells in the intestinal lining, releasing histamine and proteases that degrade tight junctions.
Reduced blood flow to the gut during prolonged stress further damages epithelial cells through oxidative injury.
Medication exposure
Common medications contribute significantly to barrier dysfunction.
NSAIDs reduce protective prostaglandins and increase epithelial cell death.
Antibiotics disrupt microbial balance and reduce short-chain fatty acid production.
Proton pump inhibitors alter digestive signaling and increase bacterial overgrowth in the small intestine.
Dysbiosis and infection
Small intestinal bacterial overgrowth, parasitic infections, and fungal overgrowth directly damage the gut lining and increase permeability. These conditions often coexist with leaky gut and must be addressed simultaneously.
Systemic consequences of intestinal permeability
The danger of leaky gut lies not in local digestion alone, but in systemic translocation.
Metabolic endotoxemia
Lipopolysaccharide from Gram-negative bacteria crosses the leaky barrier and enters circulation. Even low-grade exposure triggers chronic inflammation linked to insulin resistance, obesity, and cardiovascular disease.
Autoimmunity and molecular mimicry
Undigested food proteins and microbial antigens resemble human tissue structures. Antibodies produced against these antigens may cross-react with organs such as the thyroid, joints, or nervous system, initiating autoimmune disease.
Neuroinflammation
Inflammatory mediators generated in the gut affect the blood-brain barrier, contributing to anxiety, depression, and cognitive dysfunction.
Skin and joint manifestations
Immune complexes formed in circulation deposit in joints and skin, driving inflammatory conditions such as eczema, psoriasis, and chronic pain syndromes.
How leaky gut is diagnosed
Standard endoscopy often fails to detect barrier dysfunction because permeability occurs at a microscopic level.
Functional assessments may include:
- Lactulose-mannitol permeability testing
- Zonulin measurement in blood or stool
- Comprehensive stool analysis
- Food sensitivity testing as an indirect marker
Interpretation must always occur in clinical context.
Treatment principles for restoring gut barrier health
Healing leaky gut requires a structured, phased approach that removes ongoing insults while supporting biological repair.
Dietary modulation
Temporary removal of inflammatory triggers reduces zonulin activation and immune stimulation. Diet is individualised based on symptom patterns and test findings.
Microbiome restoration
Targeted probiotics and prebiotic fibers restore microbial signaling necessary for tight junction maintenance.
Nutrient-based repair
Specific nutrients such as L-glutamine, zinc carnosine, and polyphenols support epithelial regeneration and mucus production.
Stress and lifestyle regulation
Sleep, stress management, and nervous system regulation are essential for sustained healing.
Integrative care at ARKA Anugraha Hospital
At ARKA Anugraha Hospital, intestinal permeability is approached as a systems-level condition rather than an isolated diagnosis. Functional diagnostics, dietary assessment, and microbiome evaluation are integrated into personalised treatment plans.
Under the guidance of Dr Gaurang Ramesh, care focuses on restoring barrier integrity, reducing immune activation, and rebuilding resilience. The objective is not symptom suppression, but long-term physiological stability through evidence-based integrative gastroenterology.
Conclusion
Leaky gut syndrome is no longer a fringe concept. It represents a well-documented biological process with profound implications for immune health, metabolism, and chronic disease.
When the intestinal barrier fails, the entire body feels the impact. When it is restored, healing becomes possible across multiple systems.
Scientific understanding now supports what many patients have long experienced. The gut barrier matters, and repairing it is a legitimate, measurable, and achievable medical goal.
Frequently Asked Questions
- Is leaky gut syndrome medically recognised?
Yes. Increased intestinal permeability is well documented in medical research. - Can leaky gut exist without digestive symptoms?
Yes. It may present as fatigue, joint pain, or brain fog. - Does gluten cause leaky gut in everyone?
It increases permeability in all humans, but symptoms depend on repair capacity. - Can stress alone cause leaky gut?
Yes. Chronic stress directly disrupts tight junctions. - How long does gut healing take?
Initial improvement may occur in weeks, full repair takes months. - Are probiotics enough to fix leaky gut?
No. They must be part of a structured protocol. - Can leaky gut lead to autoimmune disease?
It is considered a prerequisite in many autoimmune conditions. - Is food sensitivity testing reliable?
It helps identify immune exposure but should not be used alone. - Does alcohol worsen permeability?
Yes. Alcohol directly damages epithelial cells. - Can children have leaky gut?
Yes, especially after infections or antibiotic exposure. - Is leaky gut reversible?
Yes, with proper intervention and compliance. - Does leaky gut affect mental health?
Yes. Gut-derived inflammation influences brain function. - Can NSAIDs cause leaky gut?
Long-term use significantly increases permeability. - Does healing require lifelong food restriction?
No. Tolerance often improves once the barrier heals. - When should medical supervision be sought?
When symptoms are chronic, multisystemic, or worsening.
Explore Arka Recipes
Discover recipes that blend taste with health, crafted by our experts for your well-being.
Arka Diagnostics
Discover groundbreaking diagnostic services exclusive to India, now available at Arka Health, Bangalore
