
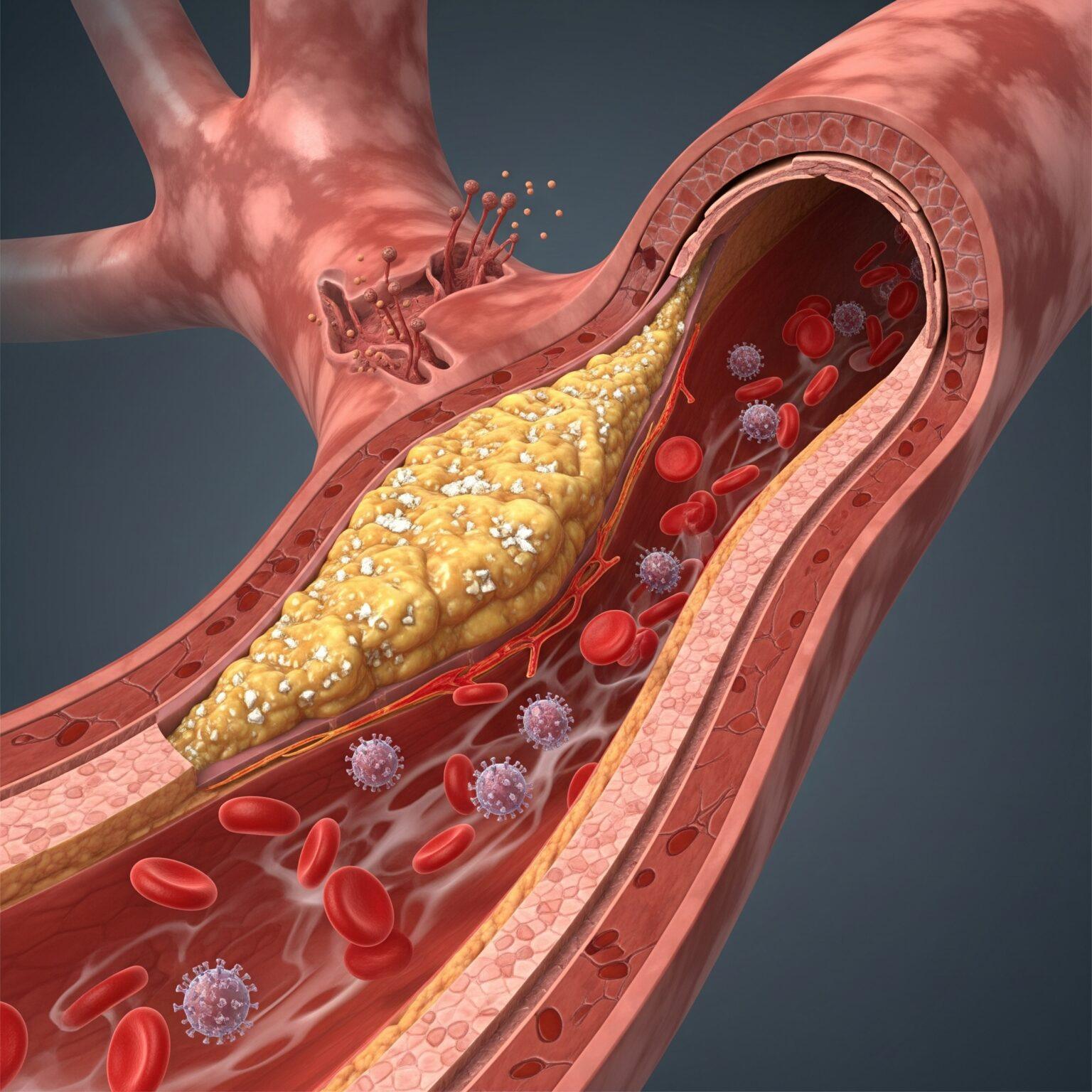
Atherosclerosis, the underlying cause of most cardiovascular diseases, has long been misunderstood as a simple plumbing problem of “clogged arteries.” Modern medicine now recognizes it as a chronic, progressive inflammatory disease, driven by a complex interplay of genetics, lifestyle, and cellular dysfunction. This new understanding opens the door for therapies that do more than just mechanically open a blockage; it calls for treatments that can address the root inflammatory cause. Enhanced External Counterpulsation (EECP) is emerging as a powerful non-invasive therapy that provides profound
EECP vascular protection by directly targeting these inflammatory pathways, offering a way to slow the progression of EECP atherosclerosis. This report explains the sophisticated anti-inflammatory mechanisms behind this innovative treatment, detailing how it shifts the vascular environment from a state of disease to one of health.
The journey of atherosclerosis begins not with a sudden blockage, but with a subtle and progressive failure of the innermost lining of the arteries: the endothelium. The endothelium is a single-cell-thick layer that acts as a dynamic and vital interface between the bloodstream and the vessel wall. In a healthy state, it is the master regulator of vascular health, maintaining endothelial homeostasis by controlling local blood flow, preventing unwanted clot formation, and actively suppressing inflammation. However, this delicate balance can be disrupted by a host of cardiovascular risk factors, including high cholesterol, hypertension, smoking, and diabetes, which inflict continuous damage and trigger a state of chronic dysfunction.
This dysfunction causes the endothelium to become “leaky” and “sticky.” One of its first failures is a reduced ability to produce nitric oxide, a critical molecule that keeps arteries relaxed, flexible, and resistant to plaque formation. As dysfunction progresses, circulating lipoproteins, particularly low-density lipoprotein (LDL) cholesterol, can more easily penetrate the arterial wall. Once trapped in this subendothelial space, these lipoproteins become oxidized, a chemical change that transforms them into potent inflammatory signals. This oxidation triggers a powerful and self-perpetuating inflammatory response. The dysfunctional endothelial cells begin to express adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), which act like molecular Velcro, capturing circulating white blood cells (monocytes) from the bloodstream. These captured monocytes then migrate into the artery wall, where they transform into aggressive macrophages and begin to relentlessly consume the oxidized lipoproteins. Over time, these macrophages become so engorged with lipids that they transform into “foam cells”—the definitive hallmark of an early atherosclerotic plaque. This entire process is a vicious cycle of lipid accumulation and chronic inflammation that thickens, hardens, and narrows the artery, setting the stage for clinical events like angina and heart attack.
The most compelling evidence for the EECP anti-inflammatory effect comes from studies demonstrating its ability to fundamentally alter the expression of the very genes that orchestrate this disease process. EECP is not just a mechanical pump; it is a biomodulatory therapy that sends powerful physiological signals to the vascular system, leading to significant and beneficial inflammatory gene downregulation.
A landmark preclinical study provided a clear window into these molecular changes. Using a porcine model of hypercholesterolemia, researchers found that a full course of EECP therapy markedly reduced the physical size of atherosclerotic lesions in both the coronary arteries and the aorta. This dramatic reduction in plaque was directly linked to a significant decrease in the accumulation of inflammatory macrophages within the vessel wall. On a genetic level, the therapy powerfully attenuated the expression of a whole suite of pro-inflammatory genes, including C-reactive protein (CRP), VCAM-1, and inducible nitric oxide synthase. Crucially, EECP was found to suppress the overactivation of the MAPK-P38/NF-kappaB signaling pathway, a central command-and-control hub that regulates the inflammatory response in endothelial cells. By quieting this pathway, EECP effectively turns down the volume on the genetic instructions that drive plaque formation.
These powerful preclinical findings have been consistently mirrored in human clinical trials. In a randomized, sham-controlled study involving patients with symptomatic coronary artery disease, a 35-session course of EECP led to significant reductions in key circulating proinflammatory cytokines. Specifically, levels of tumor necrosis factor-alpha (TNF-α), a potent inflammatory messenger, decreased by 29%, and monocyte chemoattrapctant protein-1 (MCP-1), a chemical signal that recruits more inflammatory cells to the plaque, decreased by 19% in the group receiving active EECP. The sham-treated group showed no such improvements. Another study confirmed these results, showing that EECP significantly decreased levels of TNF-α, MCP-1, soluble VCAM-1, and high-sensitivity C-reactive protein (hs-CRP). By systematically silencing this inflammatory chatter at both the genetic and protein levels, EECP helps to break the destructive cycle of
EECP atherosclerosis progression.
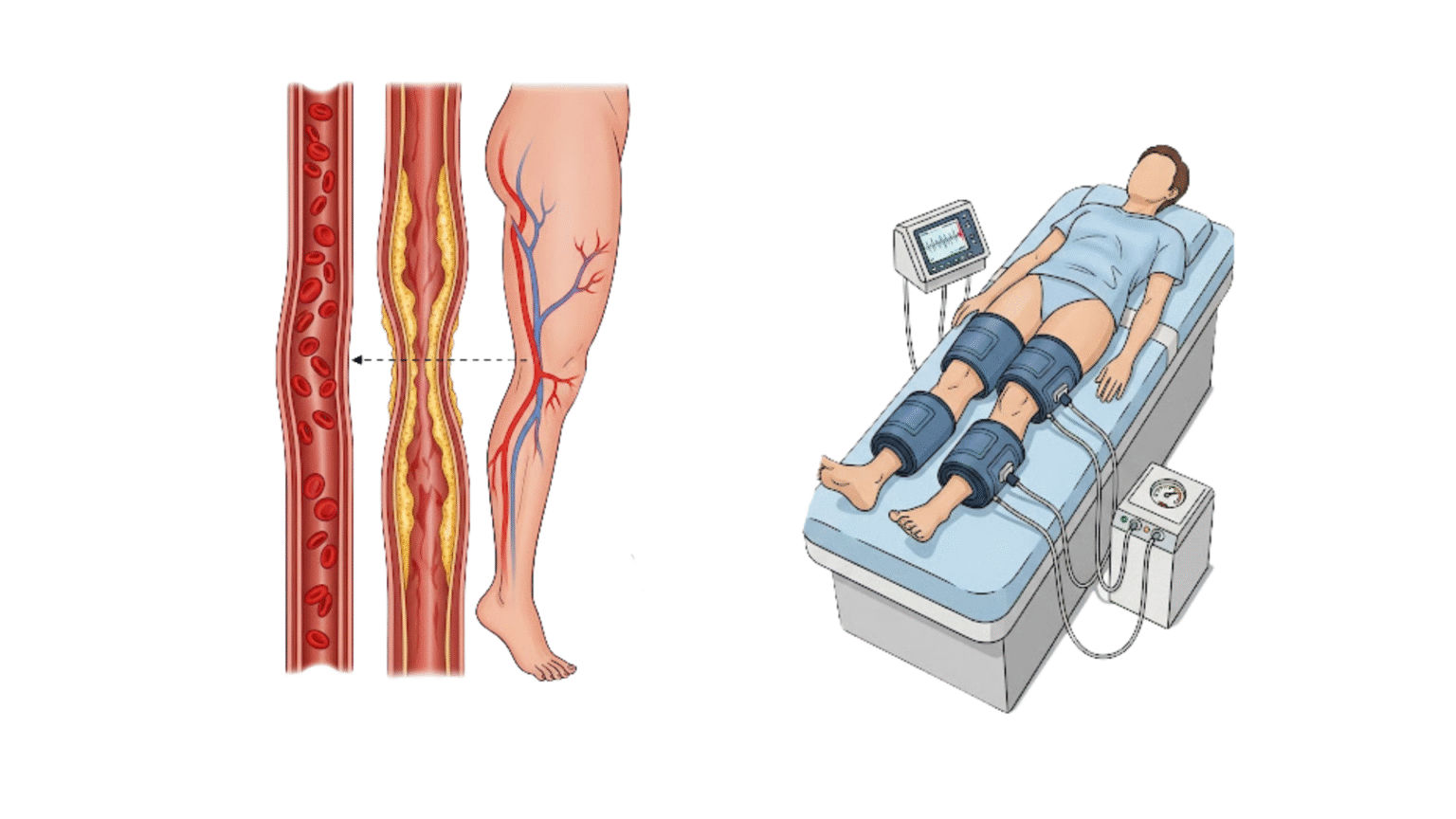
The primary mechanism by which EECP triggers this powerful anti-inflammatory cascade is through its profound and immediate effect on hemodynamics, specifically the modulation of arterial wall shear stress. Shear stress is the gentle, frictional force exerted by flowing blood on the endothelial cells lining the artery wall. This force is not trivial; it is a critical biological signal that constantly informs the endothelium about the state of the circulatory system, influencing its function and gene expression.
Atherosclerotic plaques do not form randomly. They preferentially develop in areas of low or oscillating (turbulent) shear stress, such as at artery branches and sharp curves. In these low-flow, stagnant environments, endothelial cells become dysfunctional, pro-inflammatory, and permeable to lipids. Conversely, arterial regions that are consistently exposed to high, steady (laminar) shear stress are actively protected from atherosclerosis. High shear stress is an athero-protective signal that activates genes responsible for maintaining
endothelial homeostasis, leading to the robust production of nitric oxide, the active suppression of inflammation, and the inhibition of abnormal cell growth.
EECP therapy, with its powerful, sequential cuff inflation during the heart’s diastolic phase, dramatically increases blood flow velocity and augments diastolic pressure throughout the entire arterial tree. This mechanical action directly translates into a significant and therapeutic increase in arterial wall shear stress. Studies have quantified this effect, showing that EECP can increase mean wall shear stress by over 34% and successfully convert plaque-prone, low-shear-stress environments into athero-protective, high-shear-stress environments. This sustained, pulsatile increase in shear stress, delivered consistently over the 35-hour treatment course, acts as a potent, non-pharmacological signal that effectively reprograms the endothelium, instructing it to switch from a pro-inflammatory, disease-promoting state to an anti-inflammatory, vasoprotective one
While directly measuring plaque regression in human arteries is complex and often requires invasive methods, a wealth of clinical studies provides strong indirect evidence of EECP’s anti-atherosclerotic effects. These trials consistently demonstrate significant and durable improvements in the symptoms and objective measures of the disease, reflecting a healthier, less inflamed vascular system. The landmark Multicenter Study of Enhanced External Counterpulsation (MUST-EECP) trial, a rigorous randomized, sham-controlled study, provided definitive clinical validation. The trial confirmed that patients receiving active EECP experienced a statistically significant reduction in angina frequency and a significant increase in the time to exercise-induced ischemia compared to the sham group.
These clinical benefits are a direct manifestation of improved blood flow and reduced inflammation. A comprehensive meta-analysis that pooled data from multiple studies concluded that a standard course of EECP therapy significantly increases myocardial perfusion in patients with coronary artery disease. This provides objective, imaging-based evidence that more oxygen-rich blood is successfully reaching the heart muscle, even in the presence of existing blockages. This improved perfusion is sustained long-term, with numerous studies and patient registries showing that the clinical benefits—including reduced angina and improved exercise tolerance—can last for up to five years in a majority of patients after a single course of treatment. The therapy’s ability to produce such durable relief from the symptoms of advanced
EECP atherosclerosis offers compelling clinical proof of the powerful and lasting biological changes it initiates within the vascular system.
This is the largest group who can benefit. For individuals whose symptoms occur predictably with exertion but who are not in an emergency state, EECP offers a way to manage symptoms and improve heart function without the risks of an invasive procedure.
Many individuals are not suitable candidates for angioplasty or bypass surgery due to other health issues (co-morbidities), unfavorable artery anatomy, or because they are considered too high-risk for surgical complications. For these patients, EECP is a safe and effective lifeline.
It is common for angina to return months or years after an angioplasty or even bypass surgery. EECP is an excellent option for these patients, as it works through a different mechanism to improve blood flow without performing another invasive procedure.
For individuals seeking to avoid the risks, recovery time, and potential complications of an invasive procedure, EECP provides a proven, non-surgical path to symptom management.
For clinicians managing patients undergoing EECP, monitoring the therapy’s anti-atherosclerotic benefits involves tracking both subjective patient reports and objective physiological markers of improvement. An angina diary, used to log the frequency and intensity of chest pain episodes, provides a simple yet effective way to document symptom reduction over the course of treatment. This subjective data should be complemented with objective measures. Pre- and post-treatment exercise stress tests are invaluable for quantifying improvements in exercise capacity and ischemic threshold, providing concrete evidence of enhanced cardiovascular function.
Looking ahead, the profound EECP anti-inflammatory effects suggest a much broader potential for the therapy. Future research will likely focus on using advanced, non-invasive imaging techniques, such as cardiac CT or MRI, to directly visualize changes in plaque burden or composition following a course of EECP. Furthermore, as our understanding of cardiovascular biomarkers grows, monitoring a panel of key inflammatory markers—such as hs-CRP, TNF-α, and MCP-1—could become a standard method for assessing an individual patient’s biological response to the treatment, allowing for more personalized therapy. As medicine continues to embrace the understanding of atherosclerosis as a systemic inflammatory disease, therapies like EECP that directly and non-invasively target its inflammatory core represent a vital and promising frontier in cardiovascular care.
Discover recipes that blend taste with health, crafted by our experts for your well-being.

Discover groundbreaking diagnostic services exclusive to India, now available at Arka Health, Bangalore