Dysbiosis in IBS, SIBO and Food Intolerances
A Clinical Guide to Microbiome Restoration and Root Cause Resolution

- Published on: 26/Dec/2025
- Posted By: Arka Health
Introduction: Why gut imbalance sits at the centre of modern digestive disease
For many years, digestive disorders such as irritable bowel syndrome, unexplained bloating, and food intolerances were treated as functional problems without a clear biological cause. Patients were often told their investigations were normal and advised to manage symptoms with diet changes, antispasmodics, or acid-suppressing medication. While these strategies sometimes reduced discomfort, they rarely addressed why symptoms kept returning.
Advances in microbiome research have fundamentally changed this understanding. The gut is now recognised as a complex biological ecosystem rather than a passive digestive tube. Trillions of microorganisms live within the gastrointestinal tract, shaping immune responses, regulating motility, influencing pain perception, and even affecting mood and metabolism. When this ecosystem is balanced, digestion is efficient and resilient. When disrupted, a state known as dysbiosis develops.
Dysbiosis is now understood to be a central driver behind conditions such as IBS, small intestinal bacterial overgrowth, and many so-called food intolerances. Rather than being isolated diagnoses, these conditions often represent different expressions of the same underlying imbalance. Understanding and correcting dysbiosis is therefore essential for long-term symptom resolution.
Understanding dysbiosis as a biological process
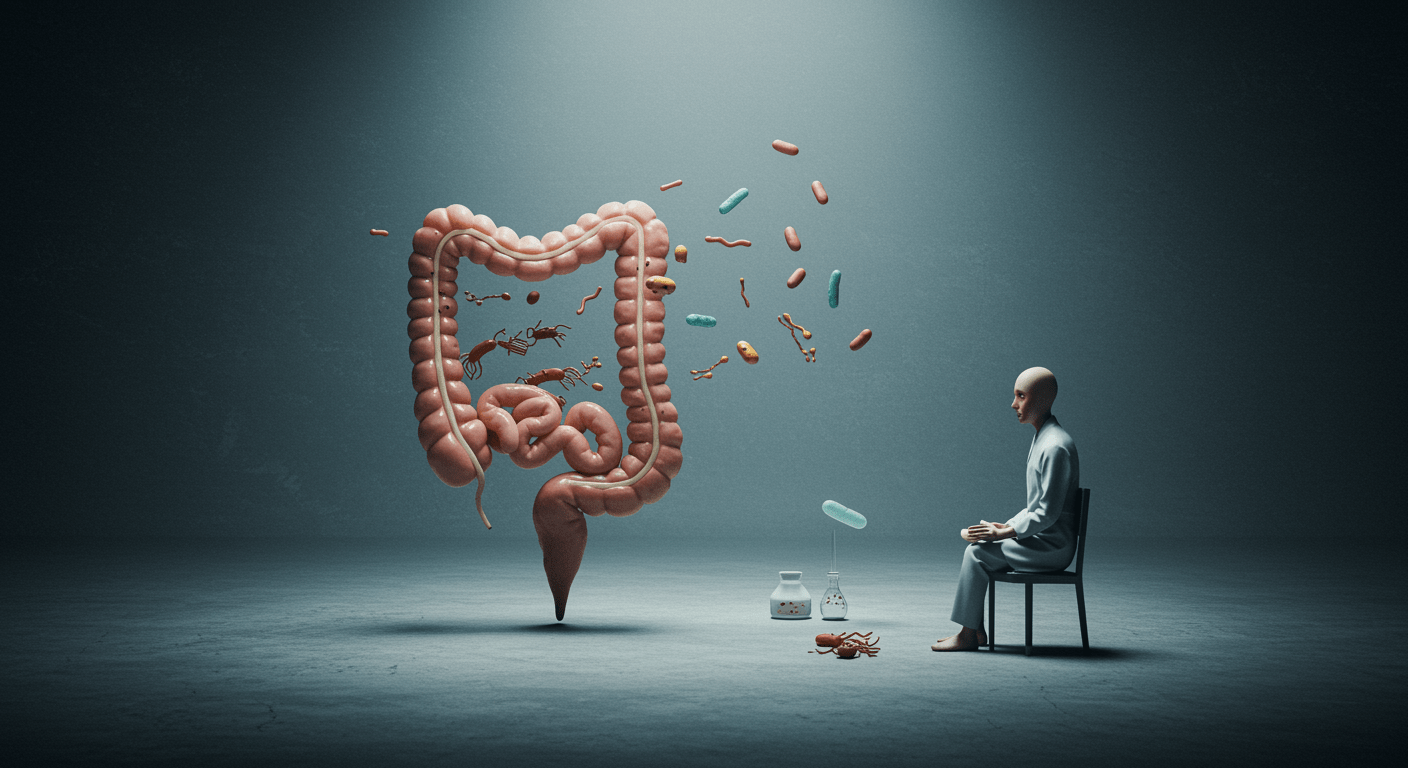
A healthy gut contains a wide range of bacterial species that coexist in balance. This diversity provides resilience, allowing the ecosystem to adapt to dietary changes, stress, or short-term illness. Dysbiosis is characterised by reduced diversity, where a small number of organisms dominate. This makes the gut more vulnerable to inflammation, infection, and metabolic instability.
Certain bacteria play a disproportionately important role in maintaining gut health. Species such as Faecalibacterium prausnitzii, Akkermansia muciniphila, and Bifidobacterium support the gut lining, regulate immune tolerance, and produce short-chain fatty acids such as butyrate. Their depletion weakens the intestinal barrier and promotes inflammation.
Pathobionts are organisms that may be harmless in small numbers but problematic when they overgrow. Species within Enterobacteriaceae or certain Clostridium groups can produce endotoxins, histamine, and gas, driving pain, bloating, and immune activation.
Why dysbiosis affects the entire body
The gut is not an isolated organ. It is closely connected to the immune system, nervous system, and endocrine signalling.
Around 70 percent of immune cells reside in gut-associated lymphoid tissue. A balanced microbiome trains these immune cells to tolerate food and beneficial bacteria while responding appropriately to threats. Dysbiosis disrupts this education, increasing immune reactivity and low-grade inflammation.
The gut also functions as a neuroendocrine organ. Most of the body’s serotonin is produced in the gut, and microbial activity influences stress hormones, appetite regulation, and motility. When dysbiosis develops, these signalling pathways become distorted, contributing to pain sensitivity, anxiety, fatigue, and altered bowel habits.
Dysbiosis and irritable bowel syndrome
IBS is one of the most common functional gastrointestinal disorders. It is defined by abdominal pain, bloating, and changes in stool consistency or frequency. While IBS has traditionally been viewed as a diagnosis of exclusion, growing evidence shows that dysbiosis plays a primary role in symptom generation.
Visceral hypersensitivity and the gut-brain axis
Many IBS patients experience pain at levels of intestinal distension that would not bother a healthy individual. This phenomenon, known as visceral hypersensitivity, is closely linked to microbial imbalance.
Dysbiotic bacteria can stimulate immune cells and sensory nerves in the gut wall. Mast cells release histamine and inflammatory mediators near nerve endings, lowering pain thresholds. Signals travel via the enteric nervous system and vagus nerve to the brain, amplifying pain perception.
Stress further worsens this process by increasing mast cell activation and altering gut motility. This explains why emotional stress often triggers IBS flares.
Post-infectious IBS
After an episode of food poisoning or gastroenteritis, some individuals never fully recover digestive stability. In post-infectious IBS, the initial infection disrupts the microbiome and damages nerve signalling involved in intestinal motility. Autoimmune mechanisms may impair the migrating motor complex, allowing bacterial overgrowth and persistent dysbiosis.
Intestinal permeability and food reactions
One of the most important consequences of dysbiosis is increased intestinal permeability, often referred to as leaky gut.
The intestinal lining is formed by a single layer of cells joined by tight junctions. Beneficial bacteria and their metabolites help maintain this barrier. Dysbiosis, particularly involving Gram-negative bacteria, increases lipopolysaccharide exposure and inflammatory signalling that weakens tight junctions.
When the barrier becomes permeable, partially digested food particles and bacterial fragments enter the bloodstream. The immune system responds by producing antibodies, leading to delayed food reactions, fatigue, brain fog, and systemic inflammation. These reactions are frequently mistaken for true food allergies.
SIBO as location-specific dysbiosis
Small intestinal bacterial overgrowth represents dysbiosis occurring in the wrong place. The small intestine is designed for digestion and absorption and normally contains relatively few bacteria. When bacteria overgrow here, fermentation occurs prematurely.
Why SIBO develops
SIBO usually arises when protective mechanisms fail. Reduced stomach acid, impaired bile flow, slowed gut motility, or damage to the migrating motor complex allow bacteria to accumulate. Stress, hypothyroidism, post-infectious changes, and long-term acid suppression are common contributors.
Gas patterns and symptoms
Hydrogen-dominant overgrowth is typically associated with diarrhoea and urgency. Methane-dominant overgrowth slows intestinal transit, leading to constipation and bloating. Hydrogen sulfide overgrowth can cause pain, diarrhoea, and heightened sensitivity but may not show clearly on standard breath tests.
Overlooked fungal overgrowth
In some cases, fungal organisms such as Candida contribute to symptoms alongside bacterial overgrowth. This is especially common after repeated antibiotic exposure. Ignoring this component can lead to incomplete treatment responses.
Why food intolerances are often secondary
Many patients believe they have developed multiple food intolerances. In reality, the gut environment is often unable to process otherwise healthy foods.
Histamine intolerance commonly arises when dysbiosis damages the brush border and reduces diamine oxidase activity. Certain bacteria also produce histamine directly, increasing the overall burden.
Fructose malabsorption can result from impaired transporter function or bacterial fermentation before absorption. Similarly, intolerance to FODMAP foods often reflects fermentation overload rather than true sensitivity.
Avoiding these foods may reduce symptoms temporarily but does not correct the underlying dysbiosis.
Systemic effects of dysbiosis
Gut imbalance has consequences beyond digestion.
The estrobolome influences estrogen metabolism. Dysbiosis can increase estrogen recirculation, contributing to hormonal imbalance, PMS, and fibroids.
The thyroid-gut axis is bidirectional. Hypothyroidism slows motility, promoting SIBO, while dysbiosis and leaky gut can trigger autoimmune thyroid disease.
Metabolic endotoxemia driven by lipopolysaccharide exposure contributes to insulin resistance, weight gain, and fatty liver disease.
Diagnosing dysbiosis accurately
Effective treatment depends on precise diagnosis.
Breath testing helps identify hydrogen and methane overgrowth patterns. Advanced stool testing using DNA-based methods assesses microbial diversity, pathogenic load, digestive capacity, and inflammatory markers.
These functional tools provide insight beyond routine investigations and allow targeted therapy.
The 5R approach to microbiome restoration
The goal is to reduce pathogenic bacteria, fungi, and dietary irritants. This may involve herbal antimicrobials, targeted medication, and temporary dietary strategies to reduce fermentation.
Digestive support such as stomach acid, enzymes, and bile is restored to improve breakdown and absorption of food.
Beneficial microbes are reintroduced strategically, using strains suited to the individual’s condition and timing.
Nutrients such as L-glutamine, zinc carnosine, and soothing botanical compounds support healing of the gut lining.
Long-term success depends on stress regulation, sleep, meal spacing, and nervous system balance.
Integrative care at ARKA Anugraha Hospital
At ARKA Anugraha Hospital, dysbiosis is evaluated and treated as a systems-based condition rather than a collection of isolated symptoms. Functional diagnostics, clinical correlation, and personalised protocols are used to identify root causes driving IBS, SIBO, and food reactions.
Dr Gaurang Ramesh combines gastroenterology with functional medicine principles, allowing structural safety alongside deeper physiological assessment. Treatment focuses on restoring balance, improving resilience, and reducing recurrence, without unrealistic claims or unnecessary restriction.
Conclusion
IBS, SIBO, and food intolerances are not random or untreatable conditions. In many cases, they are expressions of an imbalanced gut ecosystem. Dysbiosis affects digestion, immunity, hormones, and the nervous system, creating symptoms that extend far beyond the gut.
By addressing microbiome balance through targeted diagnosis and structured restoration, lasting improvement becomes possible. Healing the gut is not about eliminating foods forever or suppressing symptoms, but about restoring the environment that allows the body to function as intended.
Frequently Asked Questions
Is dysbiosis the same as IBS?
No. IBS is a symptom-based diagnosis, while dysbiosis is a biological imbalance that often causes IBS symptoms.
Can dysbiosis exist without digestive symptoms?
Yes. It may present as fatigue, brain fog, skin issues, or hormonal imbalance.
Is SIBO always caused by dysbiosis?
Yes. SIBO is a form of dysbiosis occurring in the small intestine.
Why do food intolerances keep increasing over time?
Ongoing gut barrier damage and microbial imbalance reduce tolerance.
Can probiotics worsen symptoms?
In some cases, especially with SIBO, inappropriate probiotics can increase bloating.
How long does microbiome restoration take?
Most protocols require at least 8 to 12 weeks, depending on severity.
Are antibiotics always required for SIBO?
Not always. Herbal protocols can be equally effective when chosen correctly.
Does stress really affect gut bacteria?
Yes. Stress alters motility, immunity, and microbial balance.
Can dysbiosis affect hormones?
Yes. It influences estrogen metabolism and thyroid function.
Are elimination diets a long-term solution?
No. They are tools for symptom control, not microbiome repair.
Is testing necessary before treatment?
Testing improves precision and reduces trial-and-error treatment.
Can children develop dysbiosis?
Yes. Antibiotics, infections, and diet can affect the microbiome at any age.
Explore Arka Recipes
Discover recipes that blend taste with health, crafted by our experts for your well-being.
Arka Diagnostics
Discover groundbreaking diagnostic services exclusive to India, now available at Arka Health, Bangalore
