
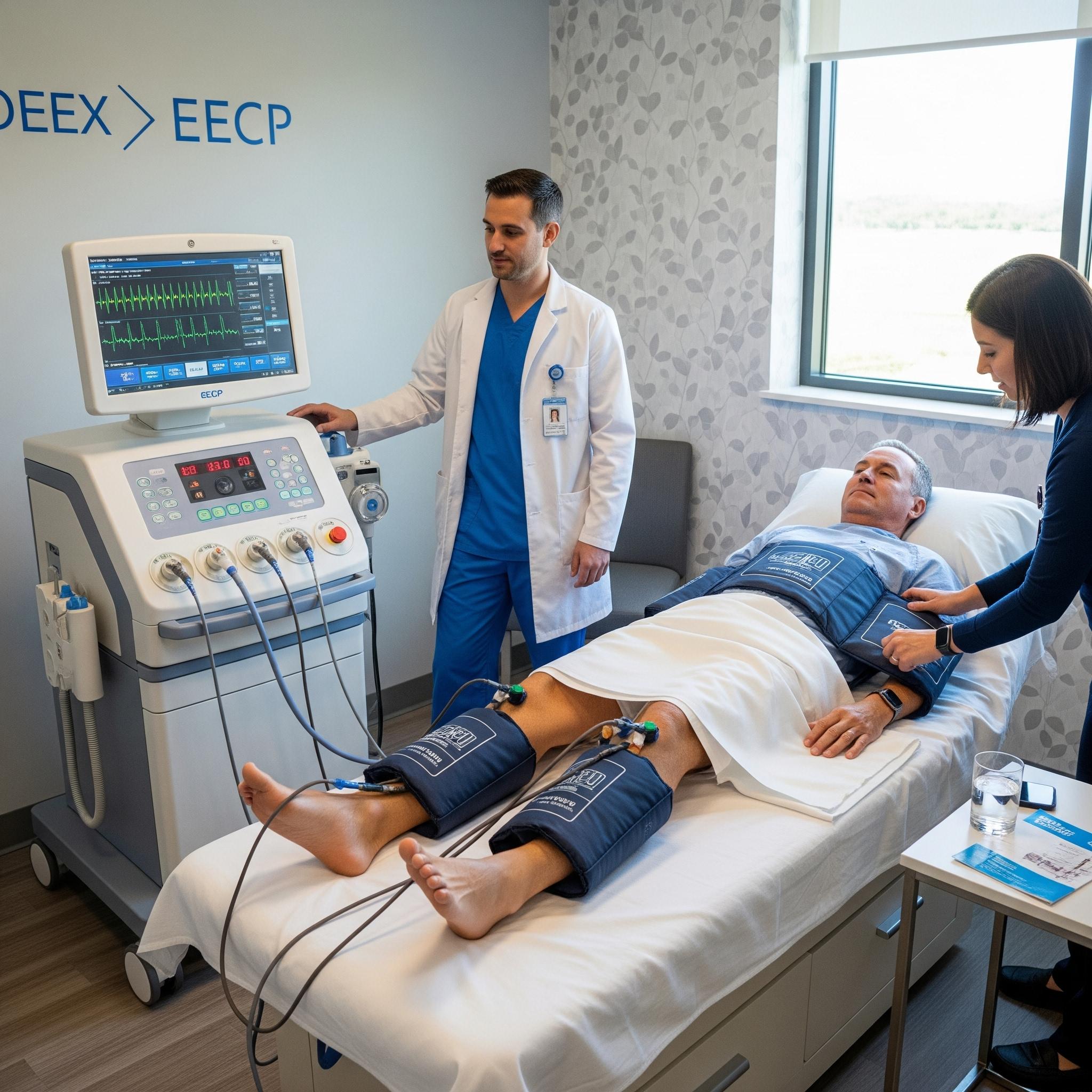
Enhanced External Counterpulsation (EECP) is a non-invasive mechanical circulatory support therapy, approved by the U.S. Food and Drug Administration (FDA) for the treatment of refractory stable angina pectoris and heart failure. While established in cardiology, its application is now being investigated for a range of other ischemic conditions, including cerebrovascular disease and renal insufficiency. The therapeutic system consists of three sets of pneumatic cuffs that are wrapped around the patient’s calves, lower thighs, and upper thighs/buttocks. These cuffs are connected to a sophisticated air compressor that is precisely timed to the patient’s cardiac cycle via electrocardiogram (ECG) monitoring. The standard treatment protocol involves a full course of one-hour sessions, typically administered once daily, five days per week, for seven consecutive weeks. This regimen is designed to induce both acute hemodynamic benefits and lasting physiological adaptations.
The fundamental therapeutic action of EECP is based on two core hemodynamic principles: diastolic augmentation and systolic unloading. This mechanism effectively functions as a non-invasive analogue to the intra-aortic balloon pump (IABP), a well-established invasive method for temporary circulatory support.
During the diastolic phase of the cardiac cycle, when the heart muscle is relaxing and filling with blood, the EECP cuffs inflate sequentially from the calves up to the thighs and buttocks. This coordinated compression squeezes a significant volume of blood from the lower extremities back into the systemic circulation. This action generates a powerful retrograde pressure wave in the aorta, a phenomenon known as
diastolic augmentation. The primary consequence of this augmented diastolic pressure is a marked increase in perfusion pressure to vital organs. While this effect is most famously leveraged to increase blood flow to the coronary arteries during diastole when myocardial perfusion is maximal, it also globally enhances perfusion in other critical vascular beds, including the carotid, renal, and hepatic arteries. Direct intracoronary measurements have shown that EECP can increase diastolic pressure by as much as 93%.
Conversely, just before the onset of ventricular contraction (systole), all three sets of cuffs deflate simultaneously and rapidly. This sudden release of pressure creates a state of reduced pressure, or a vacuum effect, in the aorta. This process, termed
systolic unloading, significantly decreases the resistance, or afterload, against which the left ventricle must eject blood. By reducing the heart’s workload, systolic unloading lowers myocardial oxygen demand, improving the overall efficiency of cardiac function.
The long-term benefits of EECP extend far beyond its immediate mechanical effects on blood pressure and flow. The therapy initiates a cascade of profound biochemical and structural adaptations within the vascular system, which are central to understanding its potential in treating stroke and kidney disease.
The most critical secondary effect of EECP is a significant increase in endothelial shear stress. The augmented blood flow velocity and increased pulsatility generated during treatment exert a greater frictional force on the endothelium, the single-cell layer lining all blood vessels. This mechanical stimulation is a powerful signal for the endothelium to modulate vascular tone and health. In response to elevated shear stress, endothelial cells upregulate the production of potent vasodilators, most notably
nitric oxide (NO) and prostacyclin. NO is a critical signaling molecule that relaxes vascular smooth muscle, leading to vasodilation, improved blood flow, and reduced inflammation. Concurrently, EECP leads to a significant reduction in the production of
endothelin-1 (ET-1), a powerful vasoconstrictor implicated in the pathophysiology of hypertension, atherosclerosis, and renal disease. Studies have documented reductions in ET-1 of approximately 25-27% following a course of EECP.
This therapy functions as a form of “passive exercise,” providing many of the hemodynamic and vascular conditioning benefits of aerobic activity without requiring physical exertion from the patient. This parallel is crucial, as it suggests EECP can offer a pathway to improved cardiovascular health for individuals who are deconditioned or unable to exercise due to conditions like post-stroke disability, severe angina, or frailty.
Furthermore, chronic exposure to increased shear stress is believed to stimulate angiogenesis (the formation of new blood vessels) and promote the development of collateral circulation. This “natural bypass” mechanism involves the upregulation of growth factors like Vascular Endothelial Growth Factor (VEGF) and Brain-Derived Neurotrophic Factor (BDNF), which support the growth and survival of new vessels and neurons, respectively. This systemic improvement in vascular health, encompassing enhanced perfusion, improved endothelial function, and the potential for new vessel growth, provides a unified physiological rationale for EECP’s emerging applications in organs beyond the heart.
For individuals seeking to avoid the risks, recovery time, and potential complications of an invasive procedure, EECP provides a proven, non-surgical path to symptom management.
Enhanced External Counterpulsation (EECP) has, for decades, offered a non-invasive lifeline to patients with debilitating angina who have exhausted other medical and surgical options. Supporters champion it as a safe, effective therapy that can restore quality of life, backed by extensive clinical use and large patient registries showing impressive, long-lasting benefits. Yet, within the medical community, an undercurrent of
EECP effectiveness skepticism persists, creating an ongoing EECP controversy. Critics question the strength of the clinical evidence, point to the potential for a powerful placebo effect, and highlight inconsistencies in trial results. This report delves into the heart of the debate, examining the arguments from both critics and supporters to provide a balanced perspective on what the science truly says.
An ischemic stroke occurs when a cerebral artery is occluded, leading to a rapid loss of blood supply to a specific brain region. This event creates a core of irreversibly damaged tissue surrounded by a larger area of functionally impaired but still viable tissue known as the ischemic penumbra. The fate of this penumbral tissue is critically time-dependent and hinges on the restoration of adequate cerebral blood flow (CBF), which is often supplied by a network of smaller, alternative vessels known as collateral circulation. Clinical evidence consistently demonstrates that poor cerebral perfusion in the aftermath of a stroke is strongly correlated with unfavorable functional outcomes, increased risk of subsequent vascular events, and higher mortality rates. Consequently, therapeutic strategies that can safely and non-invasively augment CBF and enhance collateral supply to the ischemic brain are of paramount clinical interest. EECP has been proposed as a novel method to achieve this hemodynamic goal.
Transcranial Doppler (TCD) ultrasonography, a non-invasive method to measure blood flow speed in the brain’s major arteries, has been instrumental in quantifying EECP’s effects. A landmark study involving ischemic stroke patients with large artery occlusive disease found that EECP induced a statistically significant increase in mean flow velocity in the middle cerebral arteries (MCAs), the primary vessels supplying the lateral aspects of the cerebral hemispheres. The cerebral augmentation index, representing the percentage increase in flow, was 9.64% on the side of the brain with the infarct (ipsilateral) and 9% on the opposite side (contralateral). Research from CUHK similarly reported a 9% enhancement in blood flow to both the affected and unaffected sides of the brain in stroke patients during treatment.
The carotid arteries are the main conduits of blood from the aorta to the brain. Studies using Doppler ultrasound have shown that EECP can increase blood flow volume in the common carotid artery (CCA) by 28.7%.20 More specifically for brain perfusion, one computational fluid dynamics study based on patient data indicated that EECP increased blood flow in the internal carotid artery (ICA)—the branch that directly supplies the brain—by an average of 6.67% in stroke patients.
A crucial aspect of EECP’s mechanism in stroke is its ability to transiently and safely induce hypertension. Studies show that EECP significantly increases mean arterial pressure during the one-hour treatment session, after which it returns to baseline. In a healthy individual, the brain’s intrinsic
cerebral autoregulation mechanism would cause cerebral vessels to constrict in response to this pressure rise, thereby maintaining a constant CBF. However, a key pathophysiological feature of acute ischemic stroke is the impairment or complete failure of this autoregulatory capacity.8 This pathological state becomes a therapeutic opportunity. The compromised vasculature is unable to constrict, allowing the EECP-induced rise in systemic pressure to translate directly into increased perfusion pressure and blood flow within the brain. This effect does not occur in healthy subjects, whose intact autoregulation prevents a significant rise in CBF.8 This phenomenon demonstrates that EECP’s efficacy in stroke is paradoxically dependent on the pathological state of the post-stroke brain, making it a targeted hemodynamic intervention.
The observed hemodynamic benefits of EECP appear to translate into meaningful clinical improvements in neurological function, cognitive ability, and overall recovery. While large-scale, definitive trials are still needed, the existing evidence from smaller randomized trials, observational studies, and case series is promising.
A Cochrane systematic review that analyzed two randomized controlled trials concluded that patients with acute ischemic stroke who received ECP had a significantly higher likelihood of neurological improvement compared to control participants (Relative Risk 1.75, 95% CI 1.37 to 2.23). An observational study from China provided compelling real-world data, showing that at three months post-stroke, 70.5% of patients who completed a full 35-session course of EECP achieved a good functional outcome (modified Rankin Scale score of 0-2, indicating no or slight disability). This was in stark contrast to the 46.5% in the group that did not complete the full course and the 38.5% in the group that received standard medical therapy alone. Patients frequently report tangible improvements in mobility, coordination, balance, and independence in daily activities within the first few weeks of therapy.
Post-stroke cognitive impairment is a common and debilitating consequence. EECP shows potential in mitigating these deficits. A recent study evaluating EECP as an adjunct to atorvastatin therapy in patients with post-stroke cognitive impairment found that the combination therapy led to significantly greater improvements on the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores compared to atorvastatin alone. Further supporting this, a multi-center study led by KU Medical Center investigated ECP in a broader population with mild cognitive impairment or mild Alzheimer’s disease. The results were striking: the ECP group demonstrated an average 4.5-point improvement on the Vascular Dementia Assessment Scale cognitive subscale (VADAS-cog), an effect size twice as large as the benchmark requested by the FDA for trial approval. Another study focusing on patients with Long COVID-related “brain fog” found that EECP significantly improved composite cognitive scores on the FDA-approved BrainCheck assessment tool, with enhancements across multiple domains including executive function, attention, and processing speed. These findings suggest a robust effect on cognitive function, likely driven by enhanced cerebral perfusion.
The following table summarizes key clinical studies investigating the use of EECP in stroke recovery.
Table 1: Summary of Key Clinical Trials and Studies of EECP in Ischemic Stroke Recovery
Study / Author | Year | Study Design | Patient Population & N | Intervention Details | Key Hemodynamic Outcomes | Key Clinical/Cognitive Outcomes | Salient Conclusions |
Lin et al. (Stroke) | 2012 | Prospective Case-Control | Ischemic stroke with large artery occlusive disease (n=32) vs. healthy controls (n=20) | Single session TCD monitoring during ECP | Significant increase in mean BP. MCA mean flow velocity increased ~9-10% in stroke patients; no change in controls. | Not assessed (hemodynamic study) | ECP augments CBF in ischemic stroke by elevating BP in the context of impaired autoregulation. |
Han et al. (BMJ Open) | 2013 | Prospective Observational | Ischemic stroke patients receiving ECP (n=155) vs. medical therapy alone (n=52) | Standard 35-session ECP course | Not assessed | At 3 months, 70.5% of ECP completers had good outcome (mRS 0-2) vs. 38.5% in medical group. | Completing a full course of ECP is associated with significantly better functional outcomes at 3 months. |
Myint et al. (ESOC) | 2025 | Randomized Controlled Trial (Single-center) | Symptomatic severe intracranial steno-occlusive disease (n=122) | 35 sessions of EECP + Best Medical Therapy (BMT) vs. BMT alone | Greater improvement in cerebral vasodilatory reserve (CVR) in EECP group (6.2% vs 0.6%). | Recurrent stroke at 1 year was 6.5% in EECP group vs. 26.2% in BMT group. | EECP improves cerebral hemodynamics and significantly reduces the risk of recurrent stroke in high-risk patients. |
Lin et al. (PMC) | 2023 | Randomized Controlled Trial | Post-stroke cognitive impairment (n=60) | 36 sessions of EECP + Atorvastatin vs. Atorvastatin alone | Improved pulsatility and resistance indices in cerebral arteries. | Significantly greater improvement in MMSE, MoCA, and ADL scores in the combined therapy group. | EECP combined with atorvastatin is effective in improving cognitive function and activities of daily living after stroke. |
Moriarty et al. (KU Med) | 2023 | Randomized, Sham-Controlled Trial | Mild cognitive impairment or mild Alzheimer’s (n=190) | 35 sessions of ECP vs. sham ECP | Increased cerebral blood flow (inferred) | Average 4.5-point improvement on VADAS-cog scale vs. sham. | ECP improves cognitive function, confirming the benefit of enhancing cerebral blood flow. |
Cerebral edema, or brain swelling, is a dangerous complication following a large ischemic stroke. It is caused by the accumulation of excess fluid within the brain tissue, stemming from the breakdown of the blood-brain barrier (BBB) and the death of brain cells. This swelling increases intracranial pressure, which can compress vital brain structures and lead to severe disability or death.
The existing research material does not provide direct clinical evidence that EECP reduces post-stroke cerebral edema. This remains a critical knowledge gap and an important area for future investigation. However, based on its known mechanisms, a plausible hypothesis can be formulated. EECP is reported to accelerate the clearance of metabolic waste products from brain cells. The brain’s waste clearance pathway, known as the glymphatic system, relies on the flow of cerebrospinal fluid (CSF) through brain tissue. Recent research has shown that this system can go awry after a stroke, leading to a massive influx of CSF that drives acute edema. It is therefore conceivable that by improving overall cerebral microcirculation and perfusion, EECP could help stabilize glymphatic function, enhance the clearance of excess fluid and toxic byproducts, and thereby mitigate the severity of cerebral edema. This hypothesis is speculative and requires rigorous testing with advanced neuroimaging.
A crucial aspect of EECP’s mechanism in stroke is its ability to transiently and safely induce hypertension. Studies show that EECP significantly increases mean arterial pressure during the one-hour treatment session, after which it returns to baseline. In a healthy individual, the brain’s intrinsic
cerebral autoregulation mechanism would cause cerebral vessels to constrict in response to this pressure rise, thereby maintaining a constant CBF. However, a key pathophysiological feature of acute ischemic stroke is the impairment or complete failure of this autoregulatory capacity. This pathological state becomes a therapeutic opportunity. The compromised vasculature is unable to constrict, allowing the EECP-induced rise in systemic pressure to translate directly into increased perfusion pressure and blood flow within the brain. This effect does not occur in healthy subjects, whose intact autoregulation prevents a significant rise in CBF. This phenomenon demonstrates that EECP’s efficacy in stroke is paradoxically dependent on the pathological state of the post-stroke brain, making it a targeted hemodynamic intervention.
The ideal candidates for EECP are patients who have suffered an ischemic stroke, particularly those with evidence of large artery stenosis or occlusion and impaired cerebral perfusion. Patients with co-existing coronary artery disease may derive dual benefits for both the heart and brain. The most critical contraindication is
hemorrhagic stroke, as the therapy’s mechanism of increasing blood pressure could dangerously exacerbate bleeding. Other absolute contraindications include active deep vein thrombosis (DVT) in the lower extremities, severe and uncorrected aortic valve regurgitation (which would be worsened by diastolic augmentation), known aortic or cerebral aneurysms, and active infections or bleeding disorders. Relative contraindications that require careful management include poorly controlled hypertension (systolic BP > 180 mmHg or diastolic BP > 110 mmHg) and certain cardiac arrhythmias that can interfere with the device’s ECG triggering.
Chronic Phase (beyond 6 months): EECP remains a viable option for patients with chronic, lingering deficits or for those whose recovery has plateaued. It can be particularly useful for addressing persistent cognitive impairment or functional limitations.
The heart and kidneys share a deeply interconnected and bidirectional relationship, often referred to as the cardio-renal axis. Dysfunction in one organ can directly precipitate or worsen dysfunction in the other. This interplay gives rise to
Cardiorenal Syndrome (CRS), a clinical condition where acute or chronic dysfunction of the heart induces kidney injury (Type 1 and 2 CRS), or vice versa, where kidney disease leads to cardiac dysfunction (Type 3 and 4 CRS). The underlying pathophysiology involves a vicious cycle: poor cardiac output from heart failure reduces blood flow (perfusion) to the kidneys, leading to ischemic injury and a decline in filtration capacity. In response, the compromised kidneys retain salt and water and activate harmful neurohormonal systems (like the renin-angiotensin-aldosterone system), which in turn increase blood pressure and fluid volume, placing further strain on the already failing heart. Improving systemic circulation is therefore a key therapeutic target for breaking this cycle.
EECP therapy has demonstrated a significant and positive impact on several key measures of renal hemodynamics and function. These effects provide a strong physiological basis for its potential use in protecting and supporting the kidneys.
The fundamental benefit of EECP for the kidneys is its ability to increase perfusion. Studies have consistently shown that EECP can augment renal artery blood flow by approximately 20-21% during treatment sessions. This increased delivery of oxygenated blood helps support the metabolic activity of renal cells and enhances their ability to filter waste.
GFR is the primary metric used to assess overall kidney function. A course of EECP has been shown to improve this measure. An Indian study involving patients with advanced heart and kidney disease reported an improvement in GFR of up to 20%. A more rigorous prospective, longitudinal study followed cardiac patients for a median of 16 months after a 35-session course of EECP. It found a statistically significant increase in the mean estimated GFR from a baseline of 70.47 to 76.27 mL/min/1.73
m2. This suggests a lasting improvement in filtration capacity.
EECP acts as a natural diuretic. By increasing renal perfusion and modulating hormonal signals, it enhances the kidney’s ability to excrete waste, salt, and water. Studies in healthy volunteers have recorded a 60% increase in urine production rate and a near-doubling of sodium and chloride excretion during EECP sessions. This effect can help manage fluid overload in patients with heart and kidney failure.
The following table summarizes key clinical studies investigating the use of EECP in promoting renal health.
Table 2: Summary of Clinical Evidence for EECP in Renal Health
Study / Author | Year | Study Design | Patient Population & N | Key Renal Outcomes | Key Hormonal/Biochemical Changes | Salient Conclusions |
Werner et al. (referenced in 15) | 1999 | Experimental | Healthy volunteers (n=12) | Renal blood flow increased by 21%. Urine production increased by 60%. Sodium excretion nearly doubled. | Renin levels fell by 37%. Endothelin-1 levels fell by 27%. | EECP acutely improves renal hemodynamics and favorably modulates key vasoactive hormones. |
Ruangkanchanasetr et al. | 2013 | Prospective Observational | Chronic angina and/or heart failure (n=30) | Estimated GFR increased from 70.5 to 76.3 mL/min/1.73 m2 at 16-month follow-up. Benefit was greatest in patients with baseline GFR <60. | Trend toward decreasing NT-proBNP. | EECP provides long-term improvement in renal function, especially in patients with pre-existing CKD or heart failure (i.e., cardiorenal syndrome). |
Urbonavicius et al. | 2024 | Prospective Trial | CKD patients undergoing coronary procedures (n=280) | Incidence of Contrast-Induced Nephropathy (CIN) reduced from 6.0% (control) to 1.1% (EECP). Post-procedure eGFR was significantly higher in EECP group. | Post-procedure BUN and Creatinine were significantly lower in EECP group. | EECP has a powerful protective effect against CIN in high-risk patients with chronic kidney disease. |
Li et al. (Frontiers) | 2022 | Prospective Trial | CKD and Diabetes Mellitus (DM) patients post-PCI (n=230) | In DM patients, EECP significantly increased eGFR (41.5 to 44.0). Incidence of CIN was reduced from 16.7% (rehydration alone) to 3.8% (rehydration + EECP). | Significantly decreased BUN post-procedure in EECP group. | EECP reduces the risk of CIN in the high-risk population of patients with both CKD and diabetes. |
Beyond its mechanical effects on blood flow, EECP exerts a profound influence on the neurohormonal systems that regulate blood pressure and are pathologically activated in chronic kidney and heart disease.
EECP has been shown to reduce plasma renin levels by as much as 37%. Renin is the enzyme that initiates the RAAS cascade, which ultimately leads to the production of angiotensin II and aldosterone. These hormones cause vasoconstriction, sodium retention, and fibrosis, all of which are highly detrimental to both the kidneys and the heart. By suppressing renin, EECP effectively dampens this entire harmful pathway, mirroring the action of critical drug classes like ACE inhibitors and ARBs.
As previously noted, EECP reduces levels of the potent vasoconstrictor ET-1 by about 27%. In the kidneys, high levels of ET-1 cause constriction of the renal blood vessels, which increases intra-renal pressure, reduces blood flow, and promotes kidney damage. Lowering ET-1 helps to relax the renal vasculature and improve perfusion.
In contrast to the harmful hormones it reduces, EECP increases levels of beneficial hormones like Atrial Natriuretic Peptide (ANP). ANP is released by the heart in response to stretching and promotes the excretion of sodium (natriuresis) and water (diuresis) by the kidneys, helping to lower blood pressure and reduce fluid overload.
This dual action—mechanically forcing more blood to the kidneys while biochemically downregulating the systems that cause renal vasoconstriction and damage—constitutes a comprehensive, non-pharmacological approach to renal protection.
This is arguably the most promising application. In patients with concurrent heart and kidney failure, EECP can address both organ systems simultaneously. It improves cardiac output while reducing afterload, easing the strain on the heart. At the same time, it directly improves renal perfusion and GFR. Crucially, studies have shown that the improvement in GFR following EECP is most pronounced in patients with a baseline GFR below 60 mL/min/1.73 m2 and/or elevated cardiac stress markers (NT-proBNP)—the exact clinical profile of patients with CRS.
The evidence for EECP in this setting is particularly strong. CIN is an acute kidney injury caused by the administration of iodinated contrast dye during procedures like coronary angiography and PCI. Patients with pre-existing Chronic Kidney Disease (CKD) are at very high risk. Multiple studies have now demonstrated that adding a few sessions of EECP before and after a contrast-requiring procedure can dramatically reduce the incidence of CIN. One trial reported a reduction in CIN rates from 6.0% in the control group to just 1.1% in the EECP group. This protective effect is likely due to EECP’s ability to increase renal blood flow, which helps to rapidly flush the toxic contrast agent out of the kidneys.
Diabetes is the leading cause of CKD and kidney failure. EECP may offer a supportive role in managing these patients, especially in the early stages. Its benefits are multifactorial: it improves systemic endothelial function, which is impaired in diabetes; it has been shown to improve glycemic control and insulin sensitivity, acting as a form of passive exercise; and it directly enhances renal hemodynamics. For diabetic patients who have early signs of kidney damage, such as protein leakage in the urine (microalbuminuria), a course of preventive EECP could be beneficial in slowing disease progression.
It is important to note a potential nuance in patient selection. One study investigating EECP in patients with liver cirrhosis and associated renal dysfunction found that while the therapy increased urine output, it failed to improve GFR and actually increased renal vascular resistance. This suggests that the underlying pathophysiology of kidney injury is important. EECP appears most effective for cardiorenal issues driven by poor forward blood flow, whereas its utility in hepatorenal syndrome, which has a different physiological basis, is questionable.

A synthesis of the evidence reveals that EECP’s benefits for the brain and kidneys stem from a common foundational pathway but are expressed through distinct organ-specific interactions. The shared mechanism is the therapy’s ability to induce systemic vascular conditioning. In both cases, the primary driver is diastolic augmentation, which leads to increased perfusion pressure, enhanced blood flow, and elevated endothelial shear stress. This, in turn, improves endothelial function by increasing nitric oxide production and decreasing endothelin-1 levels, promoting a healthier, more vasodilatory vascular environment.
However, the key difference lies in how this systemic effect interacts with the local pathophysiology of each organ. In ischemic stroke, the primary benefit is acutely hemodynamic and paradoxically dependent on the pathological state of impaired cerebral autoregulation. The therapy leverages this “broken” protective mechanism to force more blood into the salvageable penumbra. In chronic kidney disease, the benefit is more of a slow-acting, restorative process. It combines direct perfusion enhancement with the favorable modulation of the chronically over-activated renin-angiotensin-aldosterone system, a key driver of progressive renal fibrosis and dysfunction.
Despite the promising evidence, EECP remains an investigational therapy for both stroke rehabilitation and the management of kidney disease. A review of clinical practice guidelines from major neurological societies—such as the European Stroke Organisation (ESO), European Academy of Neurology (EAN), and the Neurocritical Care Society (NCS)—and nephrological societies like the American Society of Nephrology (ASN) reveals no current recommendations for the use of EECP in these contexts. This absence from established guidelines is the single greatest barrier to its widespread clinical adoption and reimbursement by insurance providers.
The primary reason for this status is the nature of the existing evidence. While physiologically compelling and positive in its findings, the research is largely composed of smaller single-center trials, observational cohort studies, and investigations focused on surrogate endpoints (e.g., blood flow velocity, GFR) rather than hard clinical outcomes (e.g., long-term disability, prevention of end-stage renal disease). To move from a promising concept to a standard of care, large-scale, multicenter, sham-controlled randomized clinical trials are urgently needed to definitively prove its efficacy and cost-effectiveness.
From the patient’s viewpoint, EECP offers a non-invasive and generally safe treatment experience. The procedure is typically described as comfortable, with some patients likening the sensation of the cuff inflation to a deep-pressure massage. The most common side effects are minor and related to the equipment, such as skin irritation, bruising, or muscle soreness in the legs.
The most significant drawback is the substantial time commitment. The standard 35-hour protocol requires daily visits for seven weeks, which can pose a significant logistical and financial burden for patients and their families, particularly for stroke survivors who may have mobility challenges and require transportation assistance. Despite this commitment, patient testimonials and quality-of-life assessments consistently report subjective benefits, including increased energy levels, reduced fatigue, improved exercise tolerance, and an overall enhanced sense of well-being. While anecdotal, this qualitative data provides important context to the objective clinical metrics and highlights the therapy’s potential to improve aspects of health that are highly valued by patients.
Enhanced External Counterpulsation represents a novel and highly promising non-pharmacological, non-invasive adjunctive therapy for select patient populations with ischemic stroke and chronic kidney disease. Its unique ability to provide systemic vascular conditioning through a combination of powerful hemodynamic and biochemical mechanisms addresses the core pathophysiology of ischemia and organ hypoperfusion.
For clinicians, EECP should be considered on a case-by-case basis for complex patients where conventional options are limited, have failed, or carry excessive risk. This includes ischemic stroke survivors with documented perfusion deficits whose functional recovery has plateaued, and patients with cardiorenal syndrome who are difficult to manage with standard medical therapy. The strong evidence for preventing contrast-induced nephropathy suggests that EECP could be readily integrated into the peri-procedural care of high-risk CKD patients undergoing coronary interventions.
For researchers, the path forward is clear. There is a critical need for rigorously designed, multicenter, sham-controlled randomized trials. For stroke, these trials must focus on long-term functional outcomes, such as the modified Rankin Scale, and cognitive recovery. For kidney disease, trials should evaluate hard endpoints like the progression to end-stage renal disease, the need for dialysis, and major adverse cardiovascular events in the CKD population.
In conclusion, EECP therapy leverages fundamental principles of physiology to offer a systemic benefit that could potentially shift the paradigm for managing complex ischemic diseases. It nourishes organs with improved blood flow and helps restore a healthier vascular environment. While further high-quality evidence is required to secure its place in mainstream clinical guidelines, EECP stands as a testament to the potential of innovative, non-invasive approaches to address some of modern medicine’s most challenging chronic conditions.

Discover recipes that blend taste with health, crafted by our experts for your well-being.

Discover groundbreaking diagnostic services exclusive to India, now available at Arka Health, Bangalore